Organisation name: Clinical Research Excellence Foundation (formerly known as ClinverseEdge)

27 January, 2026
Author : Dr Vijaykumar Gawali
Topic : INVESTIGATIONAL PRODUCT (IP) MANAGEMENT & ACCOUNTABILITY
1 Definition
Investigational Product (IP) refers to the drug, biological product, device, or placebo being tested in a clinical trial.
IP Accountability is the complete system of tracking, storing, dispensing, reconciling, returning, and destroying IP in compliance with ICH-GCP and sponsor SOPs.
2 PI & CRC Responsibilities
PI:
- oversees all IP handling
- ensures staff are trained
- reviews accountability logs
- signs reconciliation
CRC/Pharmacy Staff:
- receive, verify, and log IP
- maintain temperature control
- dispense and counsel patients
- document accountability
- handle returns
- coordinate destruction

3 Step-by-Step IP Management Process
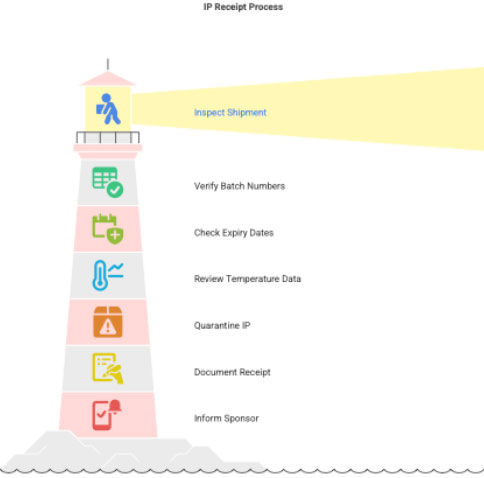
3.1IP Receipt
When shipment arrives:
- Check shipment condition
- Verify batch number, quantity, expiry
- Review shipment temperature data
- If temperature excursion → quarantine
- Document in:
- Inform sponsor immediately if excursion found
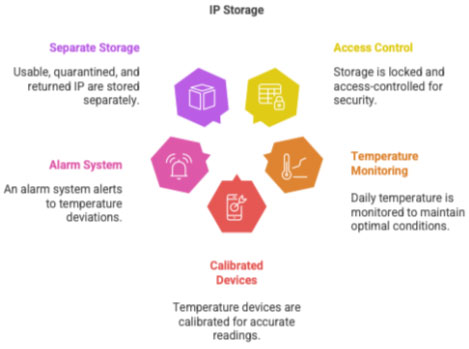
3.2IP Storage
CRC ensures:
- locked, access-controlled storage
- daily temperature monitoring (min/max)
- calibrated temperature devices
- alarm system for temperature deviations
- separate storage for:
- usable IP
- quarantined IP
- returned IP
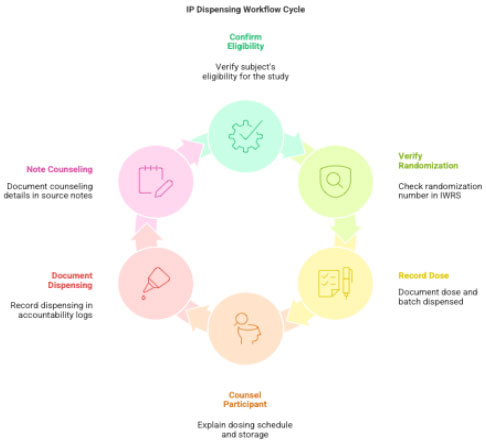
3.3 IP Dispensing
Before dispensing:
- Confirm subject eligibility
- Verify randomization number in IWRS
- Document dose, quantity, batch
- Counsel participant regarding:
- dosing schedule
- storage
- missed doses
- side-effect reporting
- return of unused IP
Document in:
- Individual IP Accountability Log
- Source notes
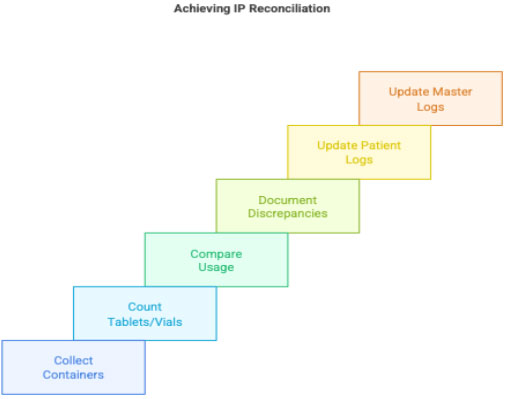
3.4 IP Reconciliation
During follow-up visits:
- CRC collects unused/empty containers
- Counts remaining tablets/vials
- Compares with expected use
- Documents discrepancies
- Updates:
- patient accountability log
- master accountability log
- IWRS
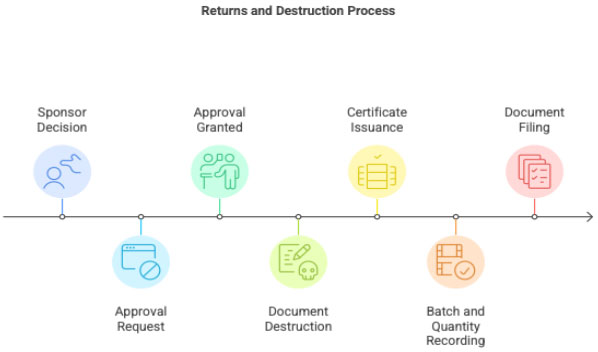
3.5 Returns and Destruction
- Sponsor decides whether IP is to be returned or destroyed
- Site cannot destroy IP without sponsor authorization
- IP destruction must be documented with:
- destruction certificate
- batch numbers
- quantity
- signatures of responsible staff
All records filed in ISF.
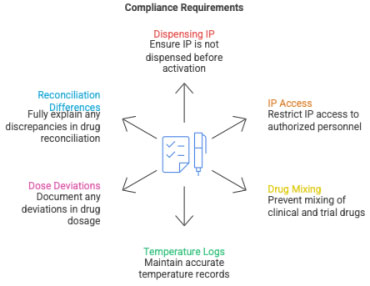
4 Compliance Requirements
- IP must NEVER be dispensed before SIV activation date
- Access must be limited to delegated personnel
- No mixing of clinical & research drug supplies
- Temperature logs must be contemporaneous
- Deviations (e.g., missed doses) must be reported and documented
- IP reconciliation differences must be explained
- IP expiry changes must be tracked
Get article by Clinical Research Excellence Foundation Experts
Organisation name: Clinical Research Excellence Foundation (formerly known as ClinverseEdge)







