Organisation name: Clinical Research Excellence Foundation (formerly known as ClinverseEdge)

27 January, 2026
Author : Dr Vijaykumar Gawali
Topic : MONITORING VISITS – SITE PREPARATION & ACTIVITIES
1 Definition
A Monitoring Visit is conducted by the Clinical Research Associate (CRA) on behalf of the sponsor to verify that:
- participant safety is protected
- trial data is accurate and verifiable
- the site is following the approved protocol and ICH-GCP
- the Investigator Site File (ISF) is complete and up-to-date
- the Investigational Product (IP) is managed correctly
Monitoring visits may be:
- Site Qualification Visit (SQV)
- Site Initiation Visit (SIV)
- Routine Monitoring Visit (RMV)
- Close-Out Visit (COV)
2 Purpose of a Routine Monitoring Visit (RMV)
- Source Data Verification (SDV)
- Source Data Review (SDR)
- Review of IP accountability
- AE/SAE review and compliance with reporting timelines
- Review of deviations, missing data, and protocol compliance
- Ensure ISF completeness
- Evaluate PI oversight
- Ensure documentation readiness for audits
3 CRC Responsibilities for Monitoring Visit Preparation
3.1 Source Document Preparation
CRC ensures:
- Source notes for every visit are complete, signed, and contemporaneous
- Lab reports, ECGs, imaging reports are filed chronologically
- Visit worksheets completed
- Eligibility documents available
- Visit windows documented
3.2 eCRF Completion
Before CRA arrives:
- All forms must be updated
- Queries should be resolved or flagged
- Missing data documented with reasons
- End-of-visit assessments verified with source
CRC should generate an internal “data completeness check” before the visit.

3.3 IP Accountability Preparation
CRC ensures:
- IP dispensing, return, and reconciliation logs are updated
- Temperature logs complete with no missing entries
- IP segregated (used, unused, quarantined)
- IWRS entries match physical stock
3.4 AE/SAE Documentation
CRC prepares:
- AE logs
- SAE Table-5 forms
- medical records (discharge summary, lab reports)
- PI causality assessments
- EC and sponsor SAE acknowledgements
The CRA will cross-check each AE/SAE with source notes, CRFs, and ISF records.
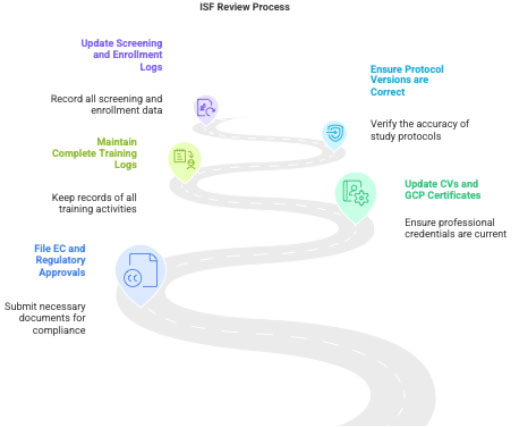
3.5 ISF Review
CRC verifies:
- Latest approvals filed
- Updated CVs, GCP certificates
- Training logs
- Protocol/SOP updates
- EC acknowledgements
- Screening/enrollment logs
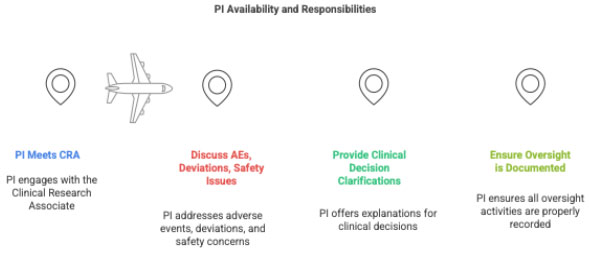
3.6 PI Availability
The PI must meet the CRA during each visit to ensure oversight.
CRC should plan:
- PI availability for 10–30 minutes
- Time to discuss SAE decisions, protocol deviations, and site issues
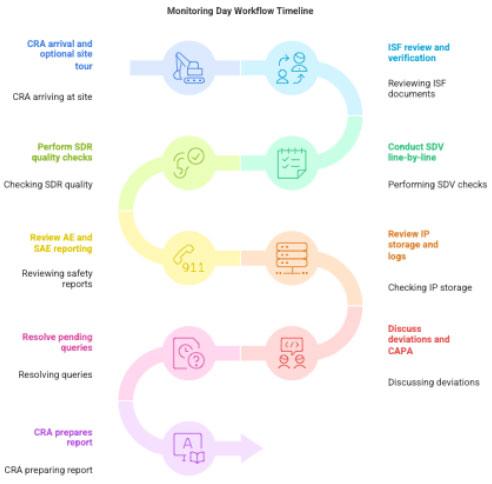
4 Step-by-Step Workflow on Monitoring Day
- CRA arrival → site tour (optional)
- Review of ISF
- SDV – line-by-line checking of source vs. CRF
- SDR – review of quality of documentation
- Review of AE/SAE reporting
- Review of IP storage, logs, and inventory
- Review of deviations & CAPA
- Review of pending queries
- PI discussion
- CRA writes report; CRC reviews next steps
Get article by Clinical Research Excellence Foundation Experts
Organisation name: Clinical Research Excellence Foundation (formerly known as ClinverseEdge)








