Organisation name: Clinical Research Excellence Foundation (formerly known as ClinverseEdge)

27 January, 2026
Author : Dr Vijaykumar Gawali
Topic : PRE-SITE INITIATION VISIT (SIV) PREPARATION
1 Definition
The Site Initiation Visit (SIV) is conducted after site selection to ensure that the site is fully prepared and trained before the first participant is enrolled.
Before SIV, the site must complete all operational, regulatory, infrastructure, and documentation readiness steps.
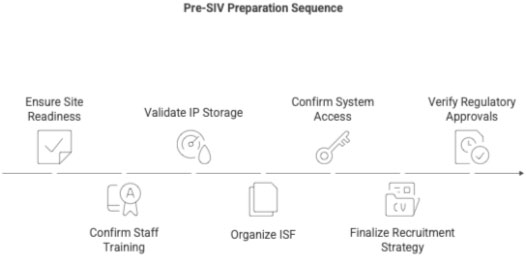
2 Purpose of Pre-SIV Preparation
- Ensure the site is ready for activation
- Ensure staff are fully trained
- Ensure IP storage conditions are validated
- Ensure ISF is complete & structured
- Ensure site systems (IVRS/IWRS/EDC) are accessible
- Ensure recruitment strategy is finalized
- Ensure regulatory readiness (DCGI, EC, SUGAM mapping)
3 Responsibilities of the CRC Before SIV
3.1 Staff Training and Coordination
CRC ensures:
- PI & all staff read the protocol
- Training meeting is conducted
- Lab staff review lab manual
- Pharmacy staff review IP instructions
- Delegation of Duties Log is prepared
- Training Log is ready for signatures
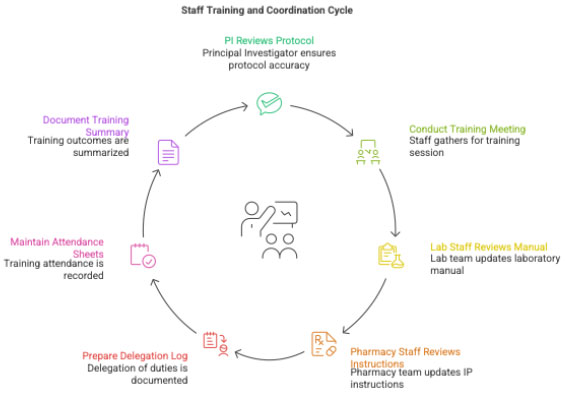
Documentation:
- Attendance sheet
- Training summary
- Delegation Log (signed by PI at SIV)
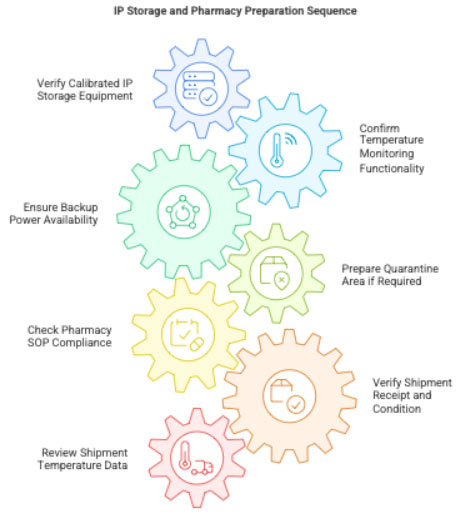
3.2 IP Storage & Pharmacy Preparation
CRC checks:
- IP cabinet/refrigerator calibrated
- Temperature monitoring systems functional
- Backup power availability
- Quarantine area ready (if required)
- Pharmacy SOP compliance
If IP has arrived:
- IP receipt acknowledged
- Shipment temperature ranges checked
- Quarantine vs. ready-for-use status documented
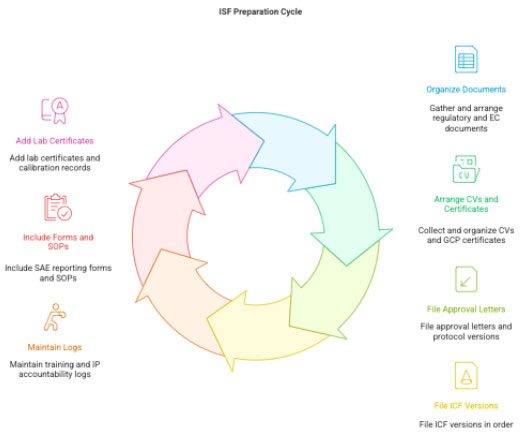
3.3 ISF Preparation (Investigator Site File)
CRC organizes:
- Regulatory & EC documents
- CVs, GCP certificates
- Approval letters, protocol versions
- ICF versions
- Training records
- IP accountability logs
- SAE reporting SOP & forms
- Lab certifications
- Equipment calibration records
Sponsor’s ISF placeholders must be replaced with original site documents.
3.4 System Readiness : Ensure logins available and tested for:
- EDC/eCRF
- IVRS/IWRS
- ePRO (if applicable)
- Sponsor safety portals
- Lab portals
3.5 Recruitment Readiness
CRC prepares:
- pre-screening plan
- clinic flow plan
- daily screening plan
- patient communication scripts (EC-approved)
- department coordination for referrals
3.6 Regulatory Readiness
CRC verifies:
- site mapped in SUGAM portal
- SAE reporting pathway validated
- compensation and medical management clarity
- EC contact details updated
4 Step-by-Step Pre-SIV Workflow
- Receive SIV agenda
- Complete ISF setup
- Conduct internal staff training
- Finalize Delegation Log
- Prepare pharmacy and IP storage
- Verify system access (EDC/IWRS/etc.)
- Prepare recruitment documentation
- Conduct final site walkthrough
- PI & CRC attend SIV
- Site activated

Get article by Clinical Research Excellence Foundation Experts
Organisation name: Clinical Research Excellence Foundation (formerly known as ClinverseEdge)







