Organisation name: Clinical Research Excellence Foundation (formerly known as ClinverseEdge)

27 January, 2026
Author : Dr Vijaykumar Gawali
Topic : PROTOCOL DEVIATIONS – IDENTIFICATION, DOCUMENTATION & MANAGEMENT
1 Definition
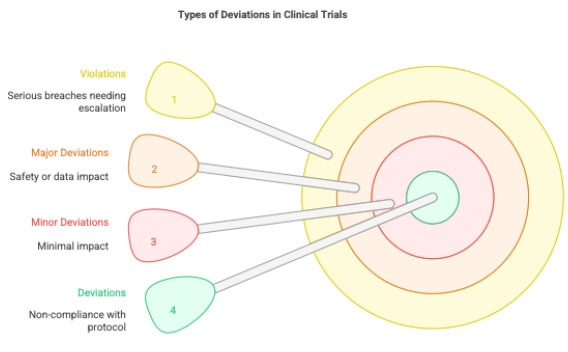
A Protocol Deviation is any non-compliance with the approved study protocol, GCP guidelines, or sponsor/CRO instructions. It may be:
Types:
- Minor deviation: Does not impact participant safety or data integrity (e.g., visit window missed by 1 day).
- Major deviation: Impacts safety, rights, welfare, or data reliability (e.g., enrolling an ineligible patient).
- Violation: Serious breach requiring immediate reporting.
ICH-GCP and NDCT Rules require timely documentation, assessment, and corrective measures for all deviations.
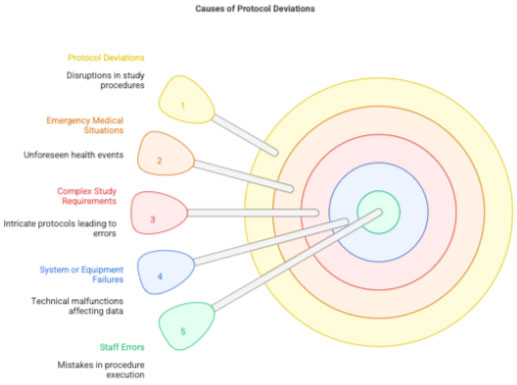
2 Causes of Deviations
- Participant-related issues (missed visits, transportation issues)
- Staff-related errors (wrong lab tests ordered)
- System failures (power outage during sample processing)
- Protocol complexity
- Emergency medical situations
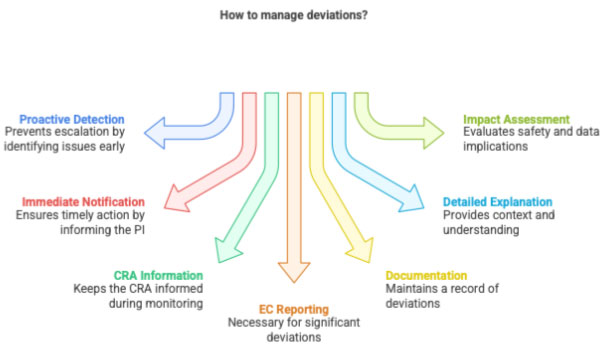
3 CRC Responsibilities in Deviation Management
3.1 Identify and Report
CRC must vigilantly detect deviations such as:
- Missed assessments
- Incorrect IP dispensing
- Out-of-window visits
- Failure to collect required samples
- Unsigned informed consent forms
CRC notifies:
- PI immediately
- CRA during monitoring
- EC if sponsor or protocol requires it
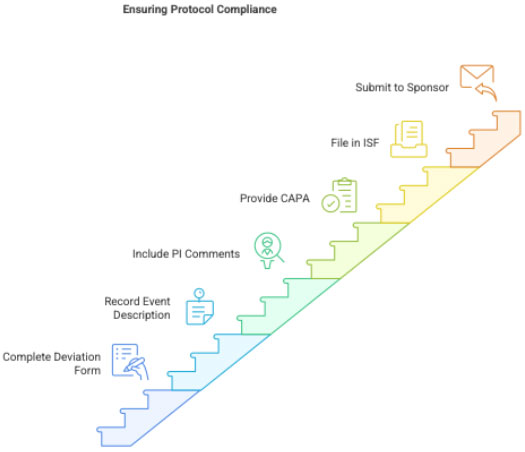
3.2 Document the Deviation
CRC prepares:
- Protocol deviation form
- Detailed explanation of what happened
- Impact assessment (does it affect safety/data?)
- PI comments/acknowledgement
- CAPA (Corrective and Preventive Action)
Filed in:
- ISF deviation section
- Submitted to sponsor
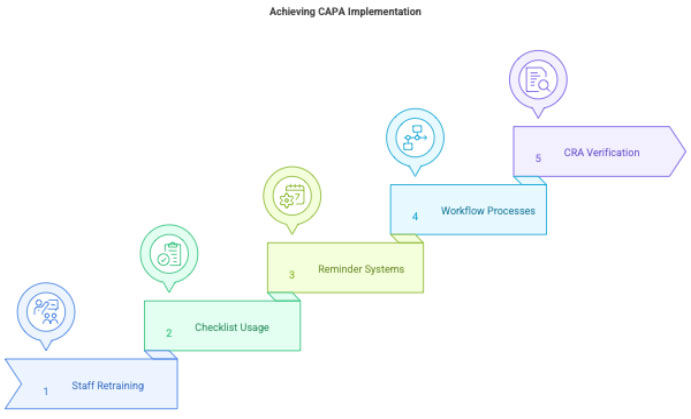
3.3 Implement CAPA
Examples:
- Staff retraining
- Creating checklist for sample processing
- Improving visit reminder system
- Updating internal process flows
CRA will verify CAPA implementation during monitoring.