Organisation name: Clinical Research Excellence Foundation (formerly known as ClinverseEdge)

28 January, 2026
Author : Dr Vijaykumar Gawali
Topic : SAFETY REPORTING – SAE SUBMISSION WORKFLOW
1 Definition
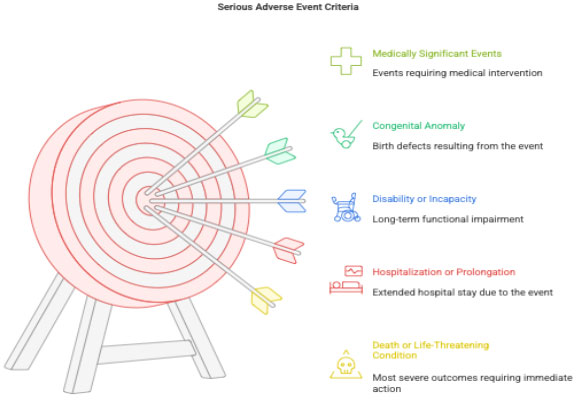
A Serious Adverse Event (SAE) is any untoward medical occurrence that:
- results in death
- is life-threatening
- requires hospitalization or prolongation of hospitalization
- results in persistent or significant disability/incapacity
- results in congenital anomaly/birth defect
- or any other medically significant event
SAEs require 24-hour reporting to:
- Sponsor
- Ethics Committee
- CDSCO (via SUGAM portal, if applicable)
- PI oversight
India’s NDCT Rules 2019 provide strict timelines for SAE reporting.
2 CRC Responsibilities in SAE Reporting
2.1 Initial 24-Hour Notification
CRC must coordinate with PI to:
- Complete Form SAE-F (Table-5)
- Gather initial medical records
- Notify sponsor within 24 hours
- Upload required documents to SUGAM (if applicable)
CRC prepares:
- initial narrative
- causality signed by PI
- event summary

2.2 Detailed Day-14 Report
Within 14 days:
- Updated SAE-F
- CIOMS form (if sponsor provides)
- Signed ICF
- Protocol version active at time of event
- Standard of care documentation (SOC)
- Updated medical records
- Treatment cost if applicable (compensation assessment)

2.3 Ongoing Follow-Up
CRC tracks:
- Additional medical reports
- Lab updates
- Clinical progress notes
- Follow-up imaging
- PI updated causality
Filed in:
- ISF SAE section
- Safety logs

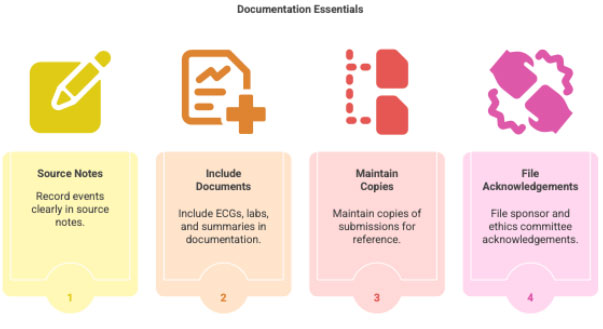
3 Documentation Requirements (Critical for CRC)
- Source notes clearly documenting onset, severity, PI assessment
- ECGs, labs, discharge summaries
- Copies of all submissions
- EC/Sponsor acknowledgements
4 Compliance Requirements
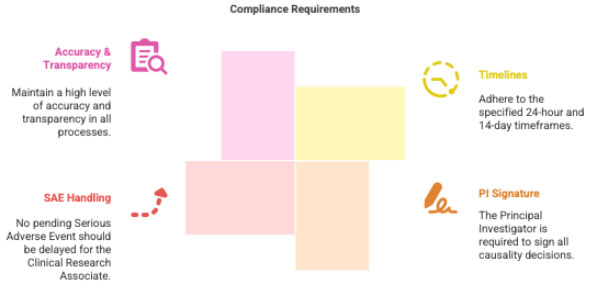
- Strict adherence to 24-hour and 14-day timelines
- PI must sign all medical causality statements
- No SAE should be kept pending for CRA review
- Maintain transparency and accuracy
Get article by Clinical Research Excellence Foundation Experts
Organisation name: Clinical Research Excellence Foundation (formerly known as ClinverseEdge)






