Organisation name: Clinical Research Excellence Foundation (formerly known as ClinverseEdge)

28 January, 2026
Author : Dr Vijaykumar Gawali
Topic : SOURCE NOTE WRITING & DOCUMENTATION PRACTICES
1 Definition
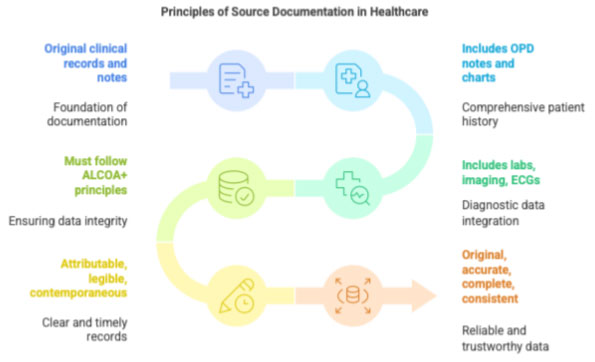
Source Documentation refers to all original clinical records and notes where trial-related information is first recorded. This includes OPD notes, inpatient charts, lab reports, imaging, ECGs, phone-call documentation, and progress notes written specifically for the trial.
Source documentation must follow the ALCOA+ principles:
- Attributable
- Legible
- Contemporaneous
- Original
- Accurate
- + Complete, Consistent, Enduring, Available
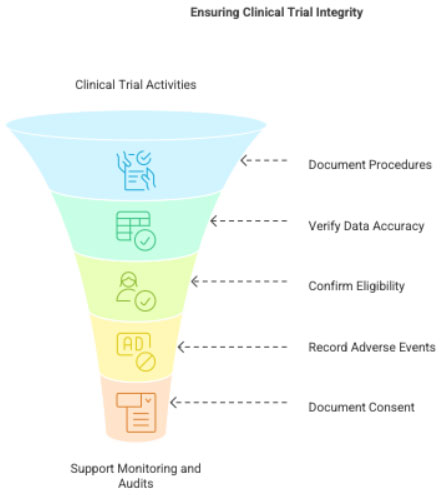
2 Purpose of Source Notes
- To document what was done during the visit
- To verify data entered into the CRF
- To ensure eligibility compliance
- To record AEs and SAEs
- To document informed consent discussions
- To support audits, inspections, and monitoring
3 CRC Responsibilities in Source Note Writing
3.1 Medical History Documentation
CRC ensures:
- Past medical history documented with supporting evidence
- Diagnosis confirmed with old medical records
- Inclusion/exclusion criteria addressed clearly
Example:
“Patient has no history of uncontrolled hypertension (BP records from last 3 months reviewed).”
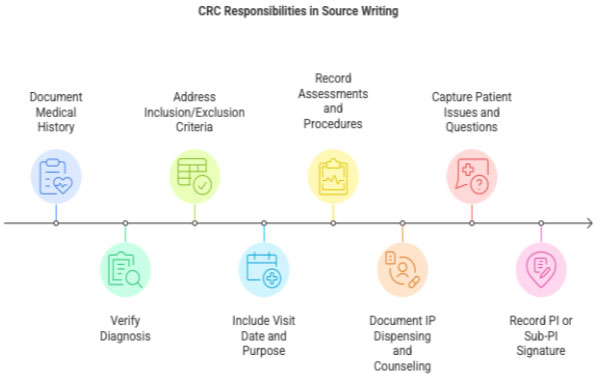
3.2 Documenting Each Study Visit
A complete source note includes:
- Visit date
- Purpose of visit (Screening/Visit 1/Visit 2/etc.)
- Assessments performed
- Procedures (vitals, ECG, labs)
- IP dispensing &counseling
- Patient questions or issues
- AE/SAE documentation (if any)
- PI/Sub-PI signature
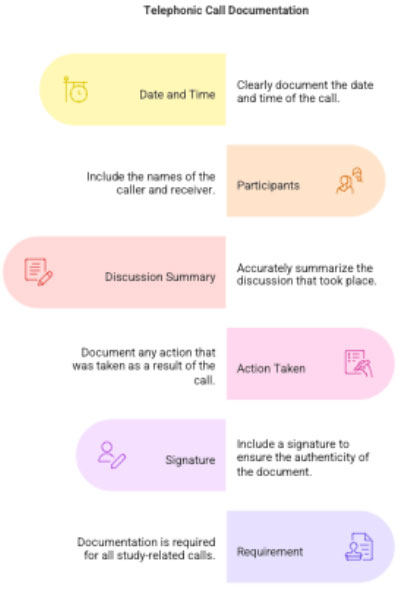
3.3 Telephonic Call Documentation
Every phone call related to the study must be documented.
Documentation must include:
- Date & time
- Caller and receiver
- Summary of discussion
- Action taken
- Signature
Example:
“20/02/2025 10:35 AM – Called patient to remind about Visit 3. Patient reports mild headache; advised to monitor. – CRC Signature”
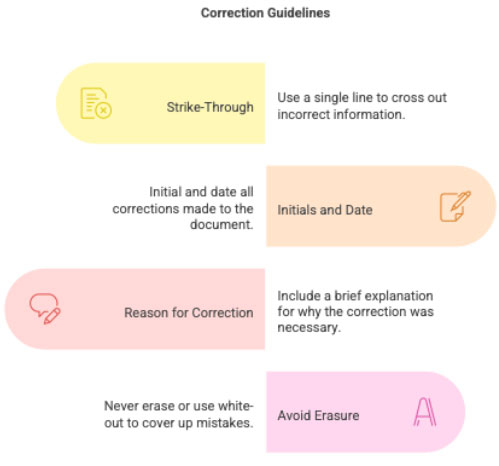
3.4 Corrections & Amendments
Corrections must follow GCP:
- Single strike-through
- Initials and date
- Reason for correction (if needed)
- No use of white-out or erasing
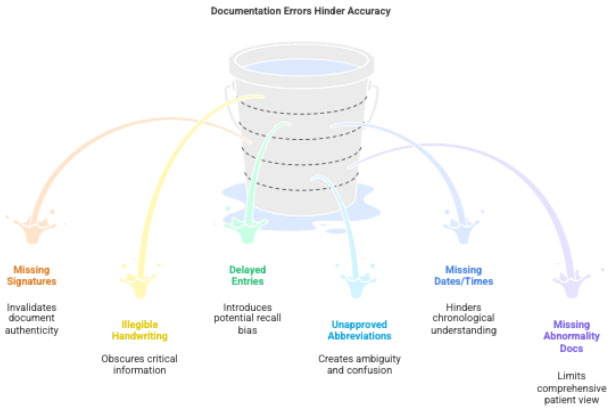
4 Common Errors to Avoid
- Missing signatures
- Unclear handwriting
- Documentation after long delay
- Using unapproved abbreviations
- Missing visit dates/timings
- Not documenting abnormal results