Organisation name: Clinical Research Excellence Foundation (formerly known as ClinverseEdge)

27 January, 2026
Author : Dr Vijaykumar Gawali
Topic : ESSENTIAL DOCUMENTS MAINTAINED IN THE INVESTIGATOR SITE FILE (ISF)
1 Definition
The Investigator Site File (ISF) is the official repository of all essential documents that demonstrate compliance with ICH-GCP, the protocol, NDCT Rules 2019, and sponsor requirements.
It must always remain organized, complete, up-to-date, and audit-ready.
2 Categories of Essential Documents in ISF
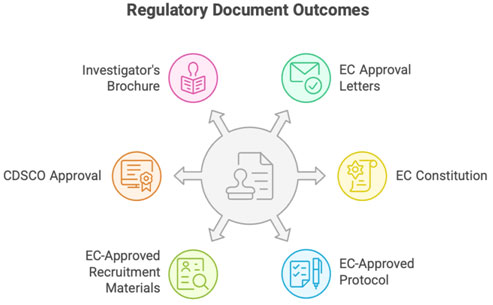
A. Regulatory & Ethics Documents
- EC approval letters (initial & amendment approvals)
- EC constitution & registration certificate
- EC-approved protocol, ICF, advertisements
- CDSCO approval/NOC (when applicable)
- IB (Investigator’s Brochure) / Product insert
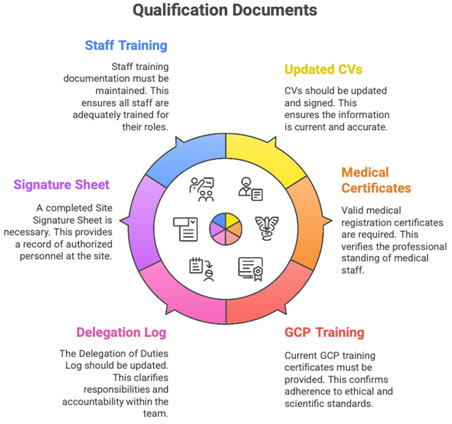
B. Investigator & Staff Qualification
- CVs (signed, updated within 2 years)
- Medical registration certificates
- GCP certificates
- Delegation of Duties Log (updated and signed)
- Site Signature Sheet
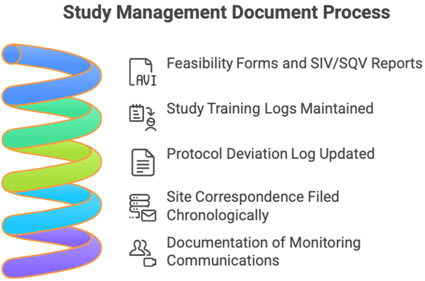
C. Study Management Section
- Feasibility form
- SIV/SQV reports
- Training logs
- Protocol deviation log
- Site correspondence file
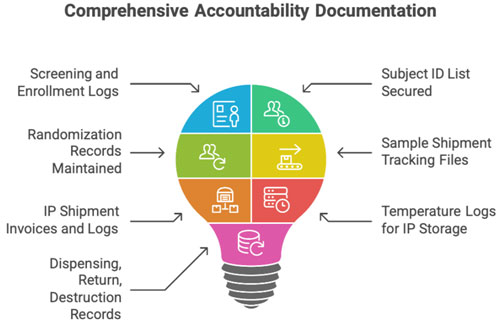
D. Participant-Related Documents
- Screening &enrollment logs
- Randomization records
- Subject ID list
- Sample shipment tracking documents
E. IP Accountability Section
- IMD/IP shipment invoices
- Temperature logs
- IP receipt, dispensing, return & destruction logs
- IWRS printouts
F. Safety Reporting Documents
- SAE forms (Table-5)
- EC & sponsor SAE acknowledgements
- SUSAR notifications
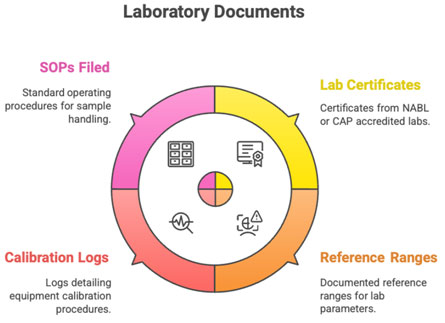
G. Laboratory/Vendor Documents
- Lab certification (NABL/CAP)
- Reference ranges
- Calibration logs
- Sample handling SOPs
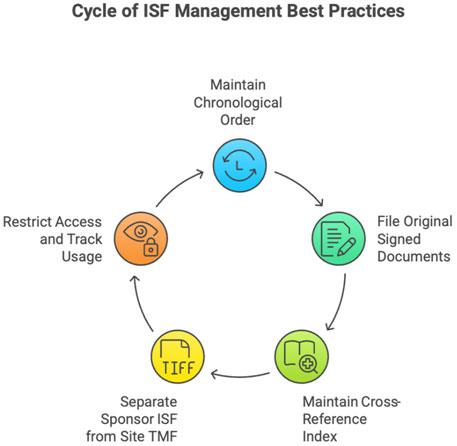
3 Best Practices for ISF Management
- Maintain chronology of document versions.
- File only original, signed documents.
- Maintain cross-referencing index.
- Separate ISF (sponsor file) from Site Trial Master File (site copy).
- Ensure limited and logged access.
Get article by Clinical Research Excellence Foundation Experts
Organisation name: Clinical Research Excellence Foundation (formerly known as ClinverseEdge)







