Organisation name: Clinical Research Excellence Foundation (formerly known as ClinverseEdge)

27 January, 2026
Author : Dr Vijaykumar Gawali
Topic : KEY RESPONSIBILITIES OF THE INVESTIGATOR DURING A CLINICAL TRIAL
1 Definition
The Principal Investigator (PI) is the medically responsible leader for the trial at the site.
ICH-GCP clearly states that PI carries full accountability for participant safety and trial integrity, although duties may be delegated.
2 PI Responsibilities Explained for CRC Understanding
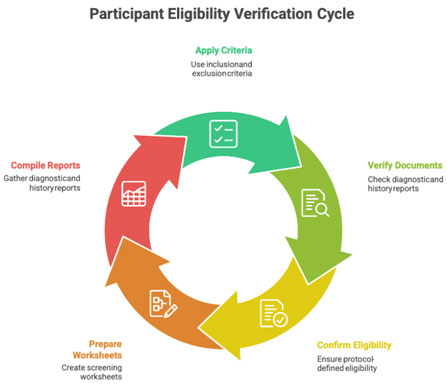
2.1 Identifying Eligible Participants
PI ensures:
- correct application of inclusion & exclusion criteria
- verification of supporting documents
- confirmation of protocol-defined eligibility
The CRC supports by preparing:
- screening worksheets
- diagnostic reports
- medical history summaries
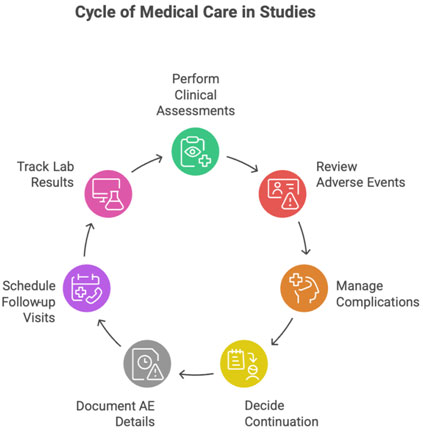
2.2 Providing Medical Care Throughout the Study
Includes:
- clinical assessments
- reviewing adverse events
- treating complications
- emergency management
- deciding on continuation/discontinuation
CRC aids by:
- documenting AE details
- scheduling follow-ups
- tracking lab results
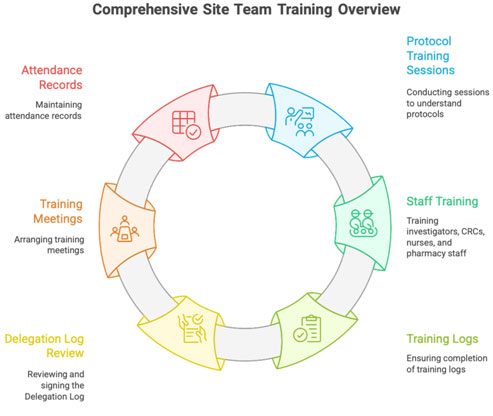
2.3 Training and Supervising Site Team
PI must:
- train sub-investigators, CRCs, nurses, pharmacy staff
- document all trainings in logs
- review and sign the Delegation Log
CRC helps:
- organize training meetings
- maintain attendance & signatures
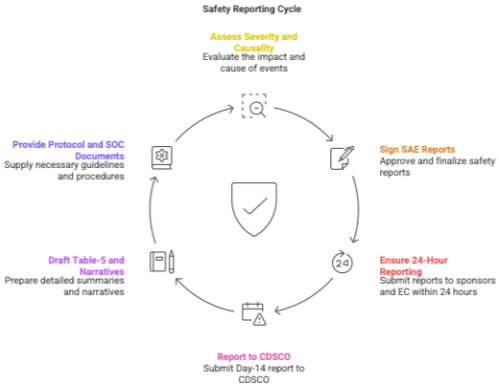
2.4 Attending to Adverse Events and Safety Reporting
PI responsibilities:
- assess severity, causality, expectedness
- sign all SAE reports
- ensure 24-hour reporting to sponsor & EC
- ensure Day-14 analysis submission to CDSCO
CRC prepares:
- Table-5
- narratives
- copies of ICF, protocol, SOC
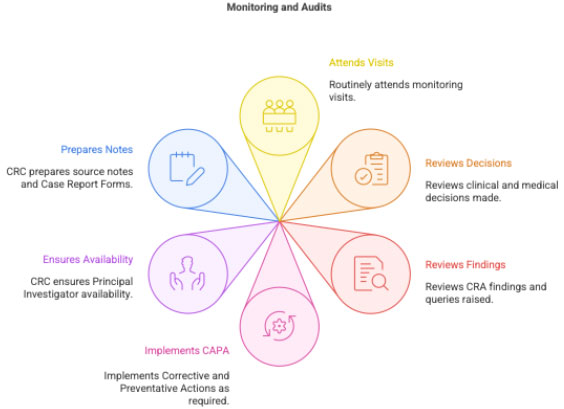
2.5 Interacting with CRA & Handling Audits
PI:
- attends monitoring visits
- clarifies medical & clinical decisions
- reviews monitoring findings
- supports CAPA implementation
CRC ensures:
- availability of PI
- source notes & CRFs ready
- ISF complete
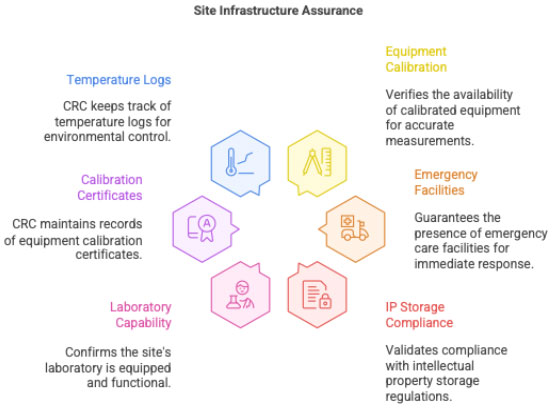
2.6 Ensuring Adequate Infrastructure
PI ensures availability of:
- calibrated equipment
- emergency care facilities
- IP storage & backup
- laboratory capability
CRC documents:
- calibration certificates
- storage temperature logs
- equipment validation records
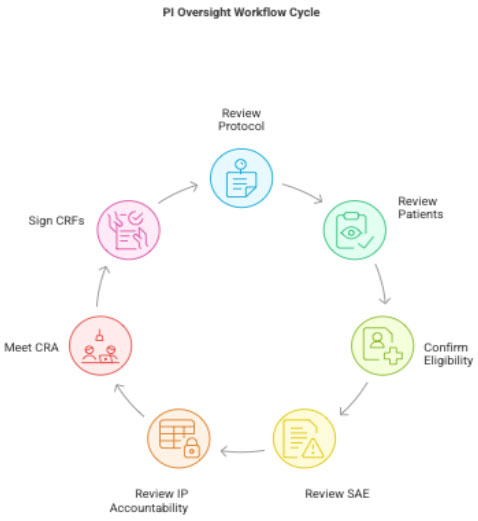
3 Step-by-Step PI Oversight Workflow (CRC Support Guide)
- PI reviews protocol → CRC arranges training
- PI reviews first patients → CRC organizes OPD screening
- PI signs eligibility → CRC prepares documentation
- PI reviews AE/SAE → CRC drafts forms
- PI reviews IP accountability → CRC provides logs
- PI meets CRA → CRC prepares agenda
- PI signs CRFs → CRC routes documents
Get article by Clinical Research Excellence Foundation Experts
Organisation name: Clinical Research Excellence Foundation (formerly known as ClinverseEdge)








